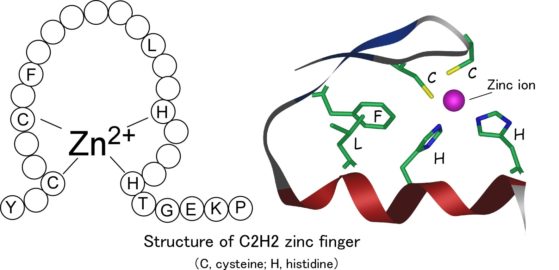
A tale of syndicate members named “gag knuckles”, “taz-two”, “hairpin”, “ribbons”, and “treble clef” is quite intriguing. Although they sound more like nicknames of a 1920’s bootlegging gang (at least to me) they are the formal nomenclature of a biochemical classification system, known commonly as zinc fingers (ZnF). The taxonomy of zinc fingers describes the morphological motif that the element creates when interacting with various molecules. Macromolecules, such as proteins and DNA, have created numerous ways to bind to other molecules and zinc fingers are one such molecular scaffold. Zinc, an essential element for biological cell proliferation and differentiation, was first isolated in 1746 by the German chemist Andreas Marggraf (1709-–1782). Zinc fingers were important in the development of genome editing, and while CRISPR remains king, zinc is making a comeback.
Proteins with zinc finger domains—meganucleases—act as molecular DNA scissors, always ready to snip and organize genetic material. The return of these biochemical bootleggers, an older generation of genome editing tools, is due to the problem of exploring the invisible molecular world of the cell. In this age of genomic editing, biologists are debating the concept of protein stability and trying to elucidate the mechanism of protein dynamics within
elaborate signalling pathways. Structural biologists imagine this process through two intermingled metaphors, the folding funnel (fig. 1) and the energy landscape (fig. 2). The energy landscape theory is a statistical description of a protein’s potential surface, and the folding funnel is a theoretical construct, a model for scientists. These two metaphors get scientists out of Levinthal’s Paradox, which argues that finding the native or stable 3-dimensional folded state of a protein, that is, the point at which it becomes biologically functional, by a random search among all possible configurations, can take anywhere from years to decades. Proteins can fold in seconds or less, therefore, biologists assert that it cannot be random. Patterns surely may be discovered. Proteins, however, no longer seem to follow a unique or prescribed folding pathway, but move in different positions, in cellular time and space, in an energy landscape resembling a funnel of irregular shape and size. Capturing the choreography of these activities is the crusade of many types of scientists, from biochemists to molecular biologists.
“Zinc Finger” Gene editing is one alternative method to CRISPR. Their scientists were the first to edit human cells and the first to conduct clinical trials with gene edited T cells. Recently, they initiated the first ever in vivo genome editing clinical trials. They have continued to perfect their zinc finger nuclease technology, which today sets the standards for genomic therapies along the three critical dimensions of specificity, precision, and efficiency.
View the rest of the blog post here with our featured blog:

Leave a Reply